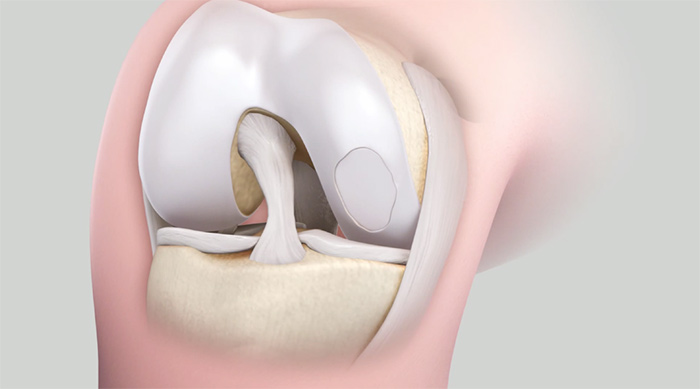
Autologous Chondrocyte Implantation (ACI)
Autologous Chondrocyte Implantation is a two-stage surgical procedure that allows you to implant your own harvested cartilage cells in an area of cartilage damage. In the first stage, the cartilage cells are biopsied from your joint and then sent to a lab for culturing and growing. This can typically be done during a diagnostic arthroscopy or other surgical procedure. Then at a later date, typically 6-8 weeks, the cells can then be implanted back into your joint in the area of cartilage damage.
In ACI, the cultured cells are implanted into an area of cartilage damage and the cells are then covered by an overlying sheath taken from your bone (periosteum) or other connective tissue.
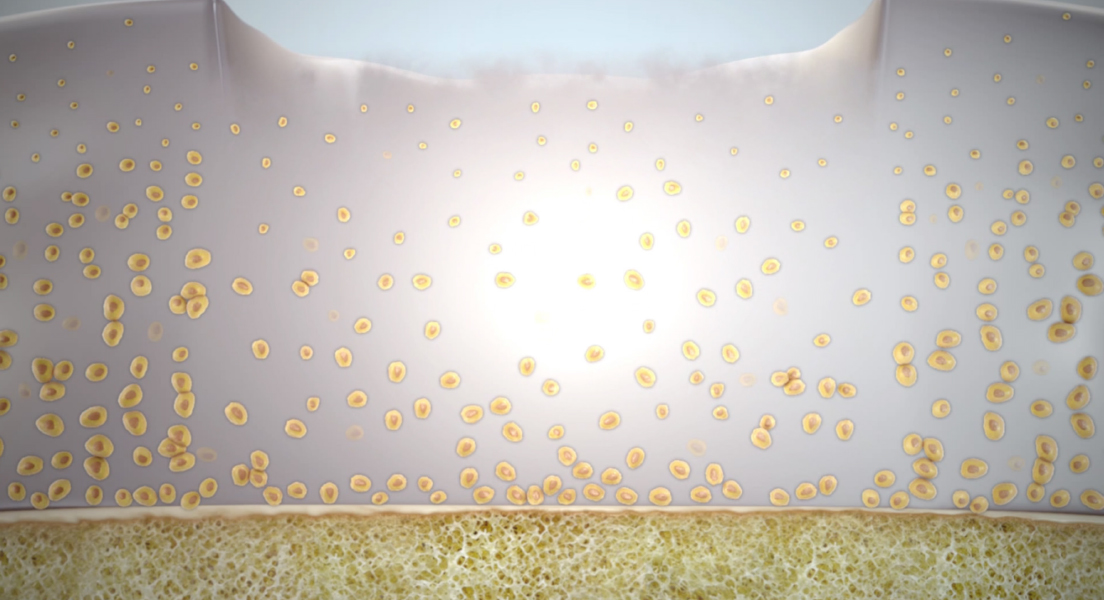
What is MACI/ Matrix-applied Autologous Chondrocyte Implantation?
The same steps for ACI also apply to MACI. However, in MACI, the cultured cells are attached to a Matrix prior to implantation so no additional tissue is necessary for implantation.

The safety and long-term clinical benefit of MACI have only been studied in the knee joint. MACI is indicated for areas of cartilage damage greater than or equal to 2 cm2. MACI has not been approved for use in joints other than the knee. The safety and effectiveness of MACI have not been proven for patients 55 years old and older. However, ultimately the damaged cartilage area size, location, and specific patient factors will determine if you are a candidate for this procedure.
What are the outcomes of ACI/ MACI?
MACI produces a type of repair tissue that alleviates symptoms and restores joint function which has been shown to form as early as 6 months after the MACI procedure. In addition, in a recent clinical trial, patients treated with MACI experienced statistically significantly greater outcomes versus microfracture, including scores related to activities of daily living, quality of life, and overall knee condition.


 ES
ES